Demonstrations Page 5 - Acids, Bases,
and Indicators
Scroll down to view photographs and short descriptions of
some of the demonstrations in the list below.
WARNING – Use at your own risk! We cannot guarantee the accuracy or the safety of these activities. Some of these activities are far more dangerous than others. The contributors and Bradley University do not assume any responsibility for these activities or their results. If you have questions, corrections, or comments please do not hesitate to contact Dean Campbell (campbell@bumail.bradley.edu) at Bradley University.
- Ferrofluid
Demonstrations
- Refrigerator
Magnet Demonstrations
- Polydimethysiloxane
Demonstrations
- LEGO® Brick Chemistry and Nanotechnology Demonstrations
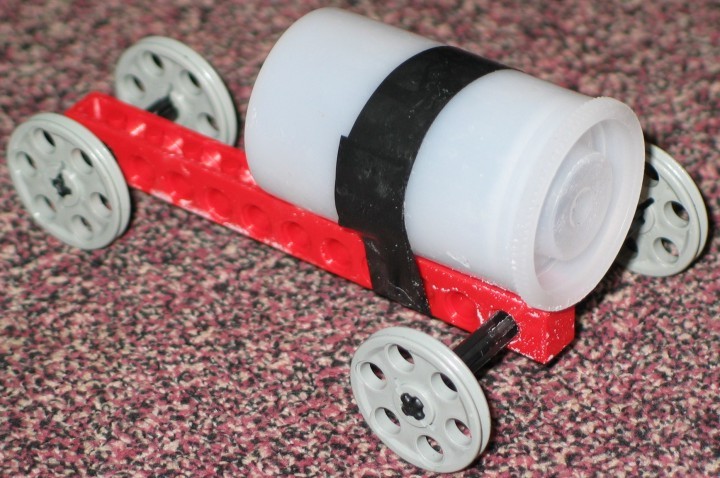
- Seltzer Popper Car
- This is a rocket car that uses the pressure from chemical
production of carbon dioxide as the basis for propulsion. The
carbon dioxide is produced by reaction between acids and carbonate
salts. The picture below shows a rocket car based on the popular
demonstration involving water and AlkaSeltzer® in a 35 mm
film canister placed on a chassis made from LEGO® parts.
The "fuel" for the popping canister demonstration is
approximately half an AlkaSeltzer® tablet, which is placed
into a 35 mm film canister (Fuji-brand film canisters seem to
work best). Water is added to the canister (to fill it approximately
one third to one half full) and then the canister is capped.
Ordinarily in the demonstration the canister is placed upright.
The citric acid and the sodium hydrogen carbonate in the AlkaSeltzer®
dissolve in the water and react to produce carbon dioxide. When
pressure builds up, the cap pops a couple of meters into the
air and spits out a quantity of fizzy water. The picture below
shows the canister taped to a frame made of LEGO® parts.
A popping canister will push a car made with this set of parts
forward 10-20 cm. Other combinations of parts may produce cars
that can move further. The challenge is to make a chassis that
is lightweight, but with a wide wheel base to prevent tumbling.
You are welcome to send alternative designs to me at campbell@bradley.edu.
WARNING: The canister cap will fly backward with considerable
speed, so a barrier to stop the cap is recommended. (The water
will also spill out of the canister when it pops open.)
-
- BELOW: A seltzer popper car.
- Thanks to Karen Campbell for passing information about this
canister demonstration from an early childhood education conference
to me and to Kristine Campbell for assistance with testing the
car. See Demonstrations Page 9 - Geology for related demonstrations, Seltzer Popper Volcanoes. More demonstrations involving LEGO® bricks may be viewed
at the site "Exploring
the Nanoworld with LEGO® Bricks."
-
- Popcorn Rocks
- When dark limestone (calcium carbonate) or dolomite (calcium
magnesium carbonate) is barely submerged in vinegar (acetic acid
solution) and the vinegar is allowed to slowly evaporate to dryness,
white popcorn-looking crystals (calcium acetate?) grow on the
surface of the rock. I have seen these rocks for sale in stores;
I found mine in a park. I have seen an article (in the Journal
of Chemical Education?) describing the chemistry of this process.
If anyone knows the reference could you please let me know?
-
- BELOW: Popcorn rock surfaces without (LEFT) and with
(RIGHT) "popcorn" crystals.


-
-
- pH Sensitivity of Colorants in Flower
Petals
- Many compounds in plants are pH sensitive and can be used
as acid/base indicators (Shakhashiri, B. Z. Chemical Demonstrations;
The University of Wisconsin Press: Madison, WI, 1989; Volume
3, pp. 50-57). For example, there have been many lab experiments
using red cabbage as a pH indicator. Some flowers can also be
used to determine the pH of a substance, and below you will see
a few examples of this. We found that pink or purple flowers
showed a large color change, but yellow flowers such as daffodils
and dandelions did not.
-
- Experiment:
-
- 1. Samples were cut into small pieces and placed into three
test tubes labeled H+, neutral, and OH-
- 2. A small amount of water was added (about 2-3mL)
- 3. 4-5 drops of concentrated hydrochloric acid or 50% sodium
hydroxide was added and the test tube was mixed
-
-
- LEFT: Quince RIGHT: Red Tulip
1.jpg)

- LEFT: Daffodil RIGHT: White Lilac


- LEFT: Dandelion RIGHT: Redbud Tree


- LEfT: Rhodadendron (fresh) RIGHT: Rhodadendron (year
old)
1.jpg)
2.jpg)
-
- LEFT: Apple Blossom RIGHT: Wild Geranium


- LEFT: Yellow Iris RIGHT: Purple Iris


- LEFT: Purple Carnation (dyed) RIGHT: Phlox (Sweet
Williams)


- LEFT: Red Cabbage RIGHT: Bee Balm


- LEFT: Peony (outer petal) RIGHT: Peony (inner petals)


-
- Other references:
- Kanda, Naoki; Asano, Takayuki; Itoh, Toshiyuki; Onoda, Makota.
Preparing "Chameleon Balls" form Natural Plants: Simple
Handmade pH Indicator and Teaching Material for Chemical Equilibrium,
J. Chem Educ. 1995 72 1131.
-
- Huntress, Ernest H. The Chemistry of the Red and Blue Pigments
of Flowers and Fruits. J. Chem Educ. 1928 5
1392, 1615.
-
- Geissman, T. A. Flower Coloration. J. Chem Educ. 1941
18 108.
-
- Dyeing Seashells with Blackberry Juice
- Seashells can be dyed by soaking in blackberry juice. The
shells below were soaked in crushed blackberries for about a
half hour. Shells that have abraded surfaces tend to turn bluish.
I interpret this as the exposed aragonite (calcium carbonate)
having a surface with a basic pH and turning the anthocyanins
in the juice blue. Some shell surfaces that are not abraded and
still have a sort of a "skin" can turn reddish. I interpret
this as the anthocyanins remaining at a more acidic pH as they
soak into this "skin".
-
- LEFT: Undyed shells. RIGHT: Dyed shells.
-


-
Return to Dr. Campbell's Favorite
Demonstrations
Last updated 1/18/12
Site created at the laboratory of Dean Campbell



1.jpg)





1.jpg)
2.jpg)















1.jpg)





1.jpg)
2.jpg)











